

When Surface Oxide measurement on a rod is high, is that because the uniform surface oxides are high or because there are sub-surface oxides present? Dual Dissolved Oxygen Level (DDOL) Testing is one method of determining this.
=
We take advantage of the discoveries from modeling sub-surface oxides. Taken together they reveal that the percentage of the test current that the sub-surface oxides draw is either independent of or somewhat inverse-dependent on the test efficiency. This means that for the everything else the same, the sub-surface oxides will draw about the same percentage of the test current whether the electrolyte allows for high or low test efficiency. An example of this phenomenon was seen in the data presented in Table III of the recent article, reproduced below:
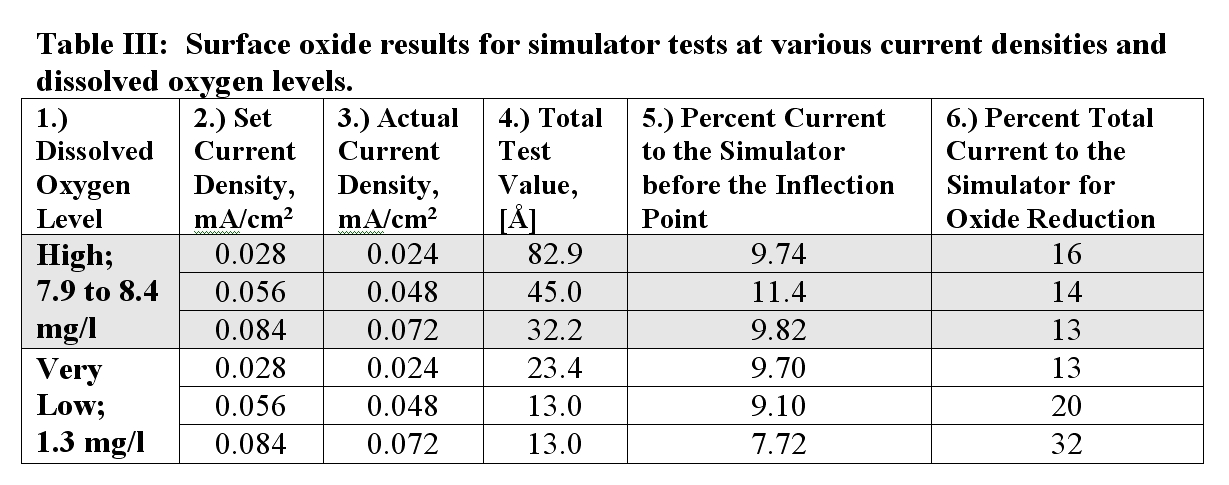
In Table III it shows that percent of the test current drawn by the sub-surface oxides for both the High DO Level test (low efficiency) and the Very Low DO Level tests (high efficiency) was about 10%. Additionally, the sub-surface oxides drew slightly more current High DO Level tests, even though less current was available because more was used to reduce the dissolved oxygen (DO). With Dual Dissolved Oxygen Level (DDOL) Testing we make use of this discovery to determine the degree of presence of sub-surface oxides.
Figure 1 shows an illustration of how current is distributed in the cell. A constant current is provided to the cell throughout the test via the electronics. A percentage of this current first is used to reduce contaminants, such as dissolved oxygen. The magnitude of this wasted current percentage is related to the amount of contaminants and the current density. After the contaminants are reduced, if subsurface oxides are present, another percentage of the supplied current is taken to reduce the subsurface oxides.
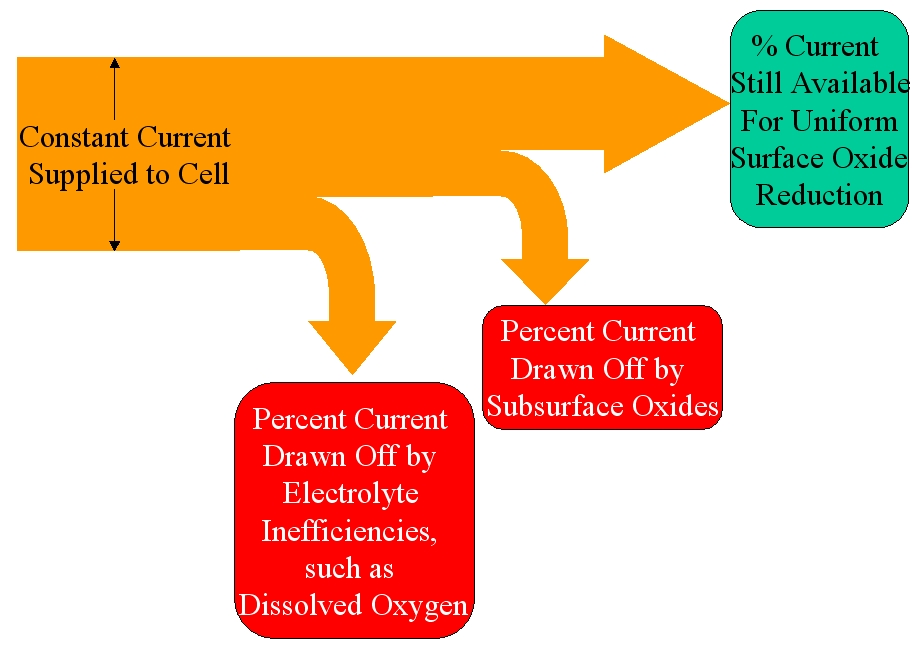
Figure 1: Illustrating the distribution of the constant current when subsurface oxides are present.
It has been discovered that an equivalent amount of subsurface oxides will draw approximately the same percentage of current, regardless of the percentage draw off by the contaminants. This is illustrated in Figures 2 and 3, below:
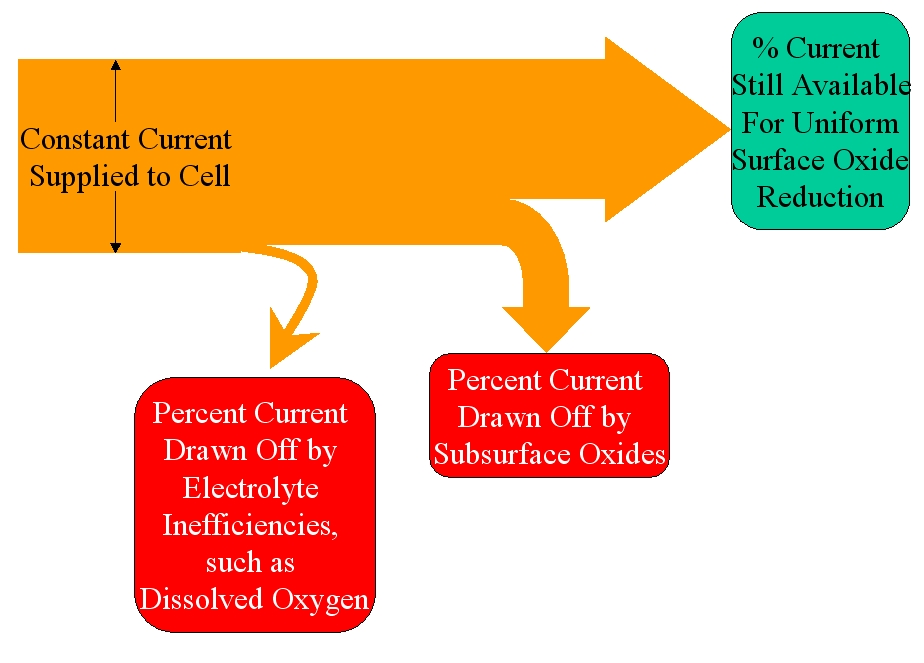
Figure 2: When electrolyte contaminants (like dissolved oxygen) are very low, subsurface oxides are far less influential.
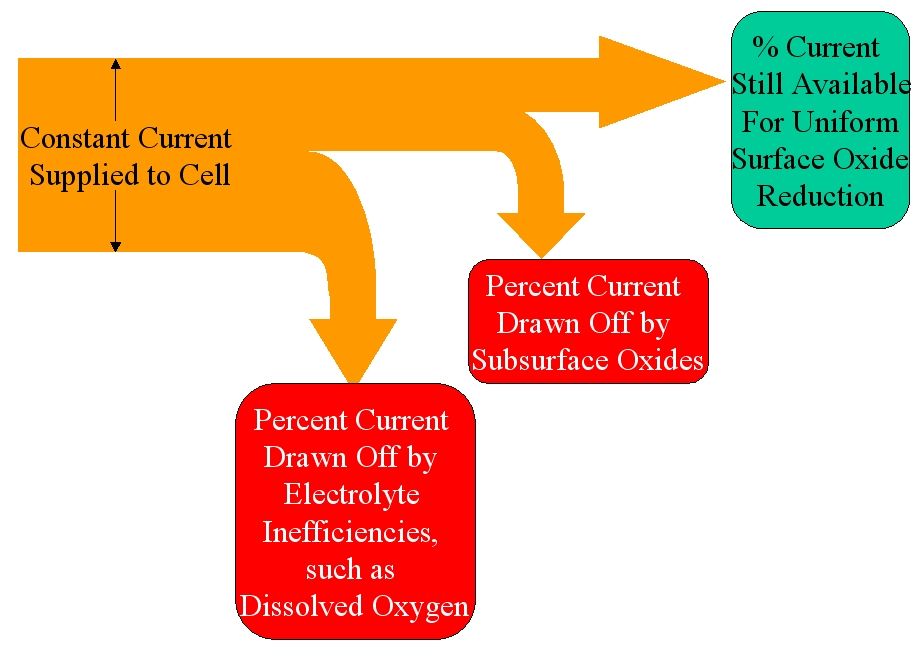
Figure 3: When electrolyte contaminants (like dissolved oxygen) are high, subsurface oxides demand a dramatic percentage of the current available for the uniform surface oxides, resulting in highly elevated test results.
Let's perform some example calculations to see how this works. Imagine that we are testing a rod which has a uniform surface oxide thickness of 100 Angstroms, but it also has sub-surface oxides present which draw 10% of the test current, similar to Table III. For testing in electrolyte which has high efficiency, as illustrated by Figure 2, let's say that only 5% of the test current goes to reducing contaminants like dissolved oxygen. So with the 5% drawn off for inefficiencies and the 10% drawn off by sub-surface oxides, 85% of the test current is available to reduce the uniform surface oxides. Thus, even though the uniform surface oxides are only 100 Angstroms, the Surface Oxide Test results would read 100 / 0.85 or 118 Angstroms, a modest increase.
Now imagine that we tested the same rod also in electrolyte that allowed only 40% efficiency, as illustrated by Figure 3. Adding 60% inefficiency to the same 10% drawn off by sub-surface oxides, only 30% of the current remains to reduce the uniform surface oxides and the Surface Oxide Test results would read 100 / 0.30 or 333 Angstroms, a dramatic increase.
The two test results just calculated are inserted into the second column of the Table below and a ratio is calculated at the last row. Also, in the last column, by similar calculations, the readings are predicted for rod also with uniform surface oxides of 100 Angstroms but with no sub-surface oxides. This results in ratios (bottom row) which are different. The lower ratio of 2.38 for rod with no sub-surface oxides can also be considered the foil ratio because the same ratio should be seen when testing copper foil (also without sub-surface oxides) in these two electrolytes. Thus, a ratio significantly higher than the foil ratio indicates the presence of sub-surface oxides. In real testing the sub-surface oxides present ratio will be even higher because slightly more current is drawn to the sub-surface oxides when test efficiency is low. Furthermore, by reducing the efficiency of the low efficiency test even further, the resultant ratio for sub-surface oxides present becomes dramatically higher than the foil ratio.
Table showing Example Calculations for Testing Rod with Uniform Surface Oxide Thickness of 100 Angstroms
| Sub-Surface Oxides Present (drawing 10% of test current) | No Sub-Surface Oxides Present | |
| High Efficiency Testing (e = 95%) | 118 Angstroms | 105 Angstroms |
| Low Efficiency Testing (e = 50%) | 333 Angstroms | 250 Angstroms |
| Ratio | 2.82 | 2.38 |
This method of determining the degree of subsurface
oxide present is implemented by testing two rod samples in separate cells with different
dissolved oxygen levels. One cell should be
maintained at the �very low� dissolved oxygen level, ~1.3 mg/l.
The second cell should be either without any type of dissolved oxygen
conditioning to provide the �high� DO levels (7.5 to 8.3 mg/l) or should be
saturated with dissolved oxygen by supplying filtered compressed air to the
deaerator. The latter method is
more precise and consistent because dissolved oxygen saturation always occurs at
a precise level for the same temperature, electrolyte concentration, and purity.
Additional
Thoughts:
|
Proper electrolyte refreshing
takes on even greater importance of for this test method, since the desire is to
control the contaminants through only the dissolved oxygen levels. | |
|
The current density setting has to be low enough to allow for a very low efficiency test, since the test efficiency is also influenced by current density as shown in Figure 4 in the recent article. | |
|
Use the Ethernet to communicate with two surface oxide testers for automatic ratio determination. |
![]()